An anchoring strategy for photoswitchable biosensor technology: Azobenzene-modified SAMs on Si(111)
| Reviews and Highlights | Quantum Science | Molecular and Soft-matter | Ultrafast Nano-optics and Nanophotonics | Mineralogy and Geochemistry |
|---|
Paul Dietrich , Fabian Michalik , Richard Schmidt , Cornelius Gahl , Guoqiang Mao , Markus Breusing , Markus B. Raschke, Beate Priewisch, Thilo Elsässer, Robert Mendelsohn, Martin Weinelt, and Karola Rück-Braun
Appl. Phys. A 93, 285 (2008).
DOI PDF
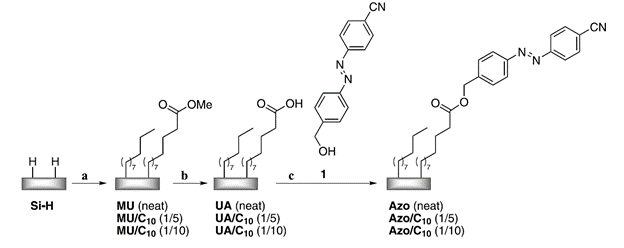
A mild and efficient procedure has been developed to obtain covalently attached self-assembled monolayers (SAMs) on Si(111) with photochromic azobenzene head-groups. Starting from neat or diluted carboxylic acid functionalized monolayers on-chip coupling reactions were applied to attach hydroxyl-functionalized azobenzene units to the SAMs by ester bond formation. The modified surfaces were characterized by high-resolution X-ray photoelectron spectroscopy (XPS), transmission Fourier transform infrared spectroscopy (FT-IR), and contact angle measurements. Reversible cis ↔ trans isomerizations of photoswitchable SAMs were monitored by wettability measurements.
