Absorption, luminescence, and Raman spectroscopic properties of thin films of benzo-annelated metal-free porphyrazines
| Reviews and Highlights | Quantum Science | Molecular and Soft-matter | Ultrafast Nano-optics and Nanophotonics | Mineralogy and Geochemistry |
|---|
Wolfgang Freyer, Catalin C. Neacsu, and Markus B. Raschke
J. Luminescence 128, 661 (2007).
DOI PDF
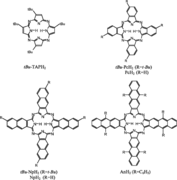
Thin film properties of the set of metal-free linearly benzo-annelated porphyrazines, tetra(tert-butyl)porphyrazine (tBu-TAPH2), tetra(tert-butyl)phthalocyanine (tBu-PcH2), tetra(tert-butyl)naphthalocyanine (tBu-NpH2), and octaphenyltetraanthraporphyrazine (AnH2) were studied by laser excitation using micro-Raman and -luminescence spectroscopy. With the exception of tBu-TAPH2 all compounds are characterized by a broad absorption pattern mainly due to excitonic coupling. A strong bathochromic energy shift for the Q-band absorption per annelated benzo-moiety was found in solution as well as in the solid state in going from tBu-TAPH2 to AnH2. Films of tBu-TAPH2, tBu-PcH2, and tBu-NpH2 exhibit a single broad featureless luminescence band with a large Stokes shift, which originates from an excitonic state. In addition, tBu-TAPH2 and tBu-NpH2 exhibit photoluminescence peaks emerging from monomer- like molecules. These porphyrazines are the only ones known to show two luminescence peaks each. A systematic decrease in luminescence efficiency of thin films is observed and can be attributed to exciton coupling and migration leading to efficient quenching of the radiative decay channels. It also allows for Raman spectroscopic characterization of the thin films and for the case of tBu-NpH2 in highly concentrated solutions.The different resonance Raman spectra of the compounds can be used as fingerprint for distinguishing the degree of benzo-annelation.
